Offering clarity to the scope of the term ‘complex medicines’
Helping innovators to develop a series of insightful, often novel characterisation techniques to understand the attributes that impact the safety and efficacy of a complex medicinal product is a key function of the Medicines Discovery Catapult.
But what would be considered within scope as a complex medicine?
Seda have worked with Medicines Discovery Catapult to offer clarity to the scope of the term ‘complex medicines’. Head over to Seda’s guest post on the MDC blog to read the full article and associated report.
From the regulators’ broad umbrella view of complex drug products (incorporating biologicals, NBCDs and hybrids of the two), the Medicines Discovery Catapult postulates that the specific technologies that would benefit from innovation and support include but are not limited to any biological or non-biological drug that falls under one or more of these categories:
Products with complex active ingredients (e.g., Drug-dendrimer conjugates, glatiramoids, polymeric compounds, antibody drug conjugates).
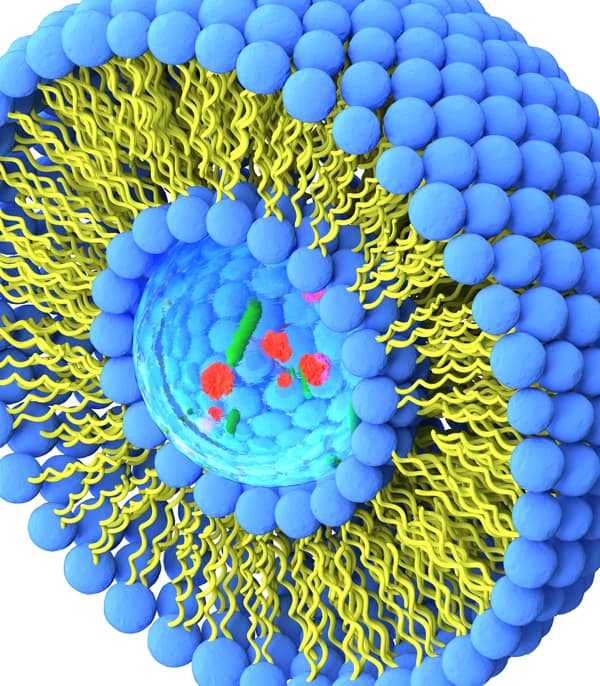
Complex dosage forms/formulations (e.g., Nanomedicines such as liposomes, polymeric/solid-lipid/inorganic nanoparticles, polymersomes, micelles, nanocrystals, colloids, microbubbles, other carriers (chitosan-based carriers for example), albumin-bound agents, extracellular vesicles (exosomes, microvesicles), extended release injectables.
Complex routes of delivery (e.g., Products with a non-systemic site of delivery, including intratumoural, targeted therapies).
Such products can present challenges for innovators and regulatory authorities alike, with established regulatory frameworks having to evolve to accommodate this ever diversifying complexity.
Find out more about Seda’s complex medicines capabilities.