Seda’s complex medicines specialists have expertise in the design, development, and regulatory authorisation of a range of complex products incorporating complex active pharmaceutical/biological ingredients, complex drug delivery systems and complex manufacturing processes.
“Complex medicines are drugs characterised by advanced formulations, delivery systems, or mechanism that present challenges in development, manufacturing and achieving regulatory approval. These may include large molecules (peptides/proteins, mAbs, nucleic acids), nanoparticulate systems and therapies requiring targeted delivery or controlled release.”
A non-exhaustive list of technologies within the scope of Seda’s offering is provided below:
- Conjugates (dendrimer, antibody, polymer-drug conjugates)
- Monoclonal antibodies
- Nucleic acid-based therapeutics (mRNA, antisense oligonucleotides, siRNA)
- Long acting injectables (depots, microspheres, hydrogels)
- Nanoparticles (liposomes, lipid nanoparticles, polymeric nanoparticles, inorganic, micelles, self-assembled systems and complexes)
- Peptide and protein complexes
What can Seda offer:
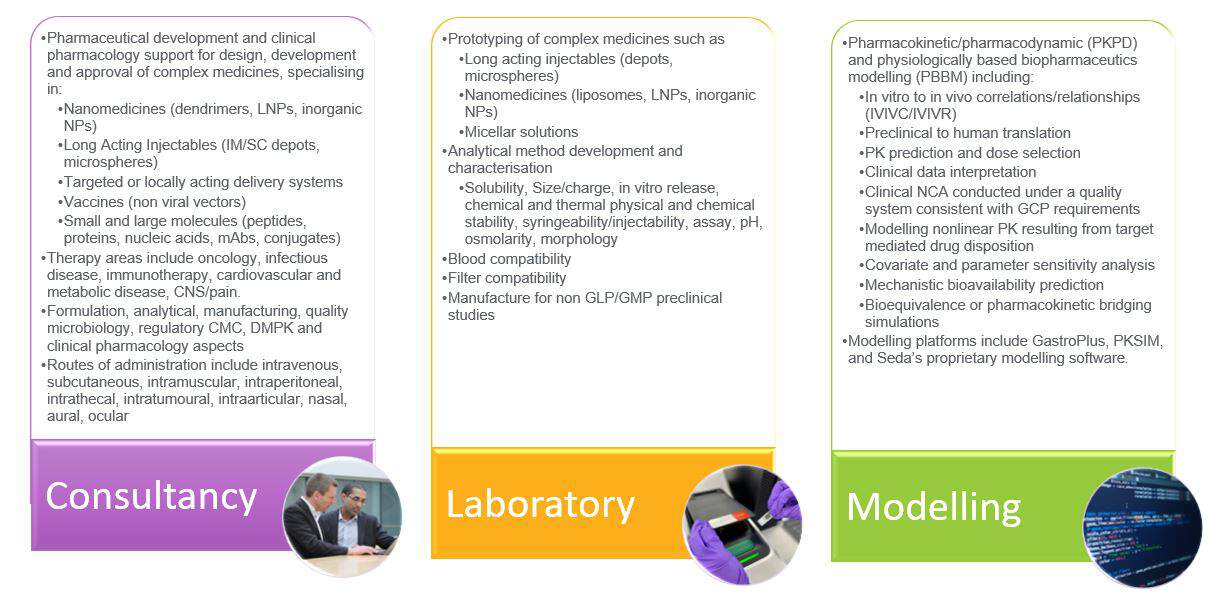
Complex medicines support can be via consultancy, laboratory, or modelling input (or any combination thereof). Take a more detailed look at our multi-faceted service offering:
Why choose Seda?
Seda’s Complex Medicines experts specialise in navigating the complexities of drug development, leveraging cutting-edge technologies and expertise to overcome the challenges unique to complex medicines. With a track record of success and a commitment to excellence, we provide tailored, technology agnostic solutions to ensure the efficient and effective development of advanced therapeutics.
Due to the intimate link between formulation properties and in vivo performance (safety and efficacy) for complex parenterals, we believe our in-house modelling, DMPK and clinical pharmacology capability significantly enhances the value of our consultancy and laboratory offerings in the complex medicines space.
Externally, we have established a network of experts in the diverse array of advanced physicochemical characterisation techniques that are so vital to success in this field. Together with close alliances to other non-clinical and clinical experts, we are ideally placed for ensuring the seamless communication required to maximise success of complex medicines, where minor changes in formulation or manufacturing process can have a major impact on performance.
Seda have no affiliations to technology platforms so Clients can be assured that all technical recommendations are always made in line with the most effective solutions.